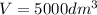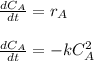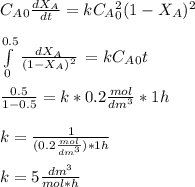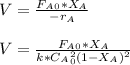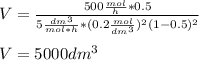Chemistry, 22.09.2020 15:01 hiyagirllyric
The elementary gas-phase reaction2A → Bis carried out in a constant-volume batch reactor where 50% conversion is achieved in 1 hour. Pure Ais charged to the reactor at an initial concentration of 0.2 mol/dm3. If the same reaction is carried outin a CSTR, what volume would be necessary to achieve 50% conversion for a feed molar flow rate of500 mol/h and an entering concentration of A of 0.2 mol/dm3? (Ans.:V = 5,000 dm3

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 22.06.2019 23:00
What prefix multiplier is appropriate for reporting a measurement of 5.57 ×10−5 m?
Answers: 1

Chemistry, 23.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 4.20 mol fe and 6.70 mol nio(oh) react?
Answers: 3
You know the right answer?
The elementary gas-phase reaction2A → Bis carried out in a constant-volume batch reactor where 50% c...
Questions

Mathematics, 18.11.2020 23:00


Chemistry, 18.11.2020 23:00


Geography, 18.11.2020 23:00

History, 18.11.2020 23:00



History, 18.11.2020 23:00



Arts, 18.11.2020 23:00



Mathematics, 18.11.2020 23:00



Mathematics, 18.11.2020 23:00


Social Studies, 18.11.2020 23:00