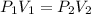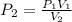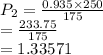Chemistry, 22.09.2020 14:01 jeffreyaxtell4542
A sample of helium gas has a volume of 250.0 mL when it pressure is 0.935 atm. If the
temperature remains constant, what will the pressure of the gas be when it has a volume of
175.0 mL?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1
You know the right answer?
A sample of helium gas has a volume of 250.0 mL when it pressure is 0.935 atm. If the
temperature r...
Questions




Mathematics, 13.03.2021 01:00

Physics, 13.03.2021 01:00


Mathematics, 13.03.2021 01:00

English, 13.03.2021 01:00


Mathematics, 13.03.2021 01:00


Mathematics, 13.03.2021 01:00

Arts, 13.03.2021 01:00


Mathematics, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00

Chemistry, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00

Biology, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00