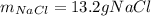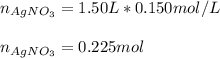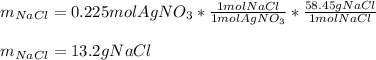Chemistry, 22.09.2020 01:01 emma042902
Calculate the mass of NaCl that must be added to completely react with 1.50 L of a 0.150 M AgNO3 solution according to the reaction below: NaCl (aq) + AgNO3 (aq) → AgCl (s) + NaNO3 (aq)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3

Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2
You know the right answer?
Calculate the mass of NaCl that must be added to completely react with 1.50 L of a 0.150 M AgNO3 sol...
Questions

Computers and Technology, 14.06.2021 07:10

Arts, 14.06.2021 07:10


Computers and Technology, 14.06.2021 07:10


Spanish, 14.06.2021 07:10

Mathematics, 14.06.2021 07:10

Chemistry, 14.06.2021 07:10

Social Studies, 14.06.2021 07:10

English, 14.06.2021 07:10


Mathematics, 14.06.2021 07:10

Mathematics, 14.06.2021 07:10

Mathematics, 14.06.2021 07:10

English, 14.06.2021 07:10

Mathematics, 14.06.2021 07:10


Mathematics, 14.06.2021 07:20