
Chemistry, 20.09.2020 19:01 miguelsanchez1456
An excited ozone molecule, O3*, in the atmosphere can undergo one of the following reactions, O3* → O3 (1) fluorescenceO3* → O + O2 (2) decompositionO3* + M → O3 + M (3) deactivation, where M is an inert molecule, the rate constant for the fluorescence reaction is k1, the rate constant for the decomposition reaction is k2, and the rate constant for the deactivation reaction is k3. Write a simplified expression for the fraction, X, of ozone molecules undergoing deactivation in terms of the rate constants. (Use the following as necessary: k1, k2, k3, cO for [O3*], and cM for [M].)

Answers: 3


Another question on Chemistry




Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1
You know the right answer?
An excited ozone molecule, O3*, in the atmosphere can undergo one of the following reactions, O3* →...
Questions

Health, 06.01.2020 11:31

Mathematics, 06.01.2020 11:31

Spanish, 06.01.2020 11:31

Mathematics, 06.01.2020 11:31

Mathematics, 06.01.2020 11:31

Mathematics, 06.01.2020 11:31




Mathematics, 06.01.2020 11:31

Mathematics, 06.01.2020 11:31

Chemistry, 06.01.2020 11:31

Mathematics, 06.01.2020 11:31





Mathematics, 06.01.2020 11:31


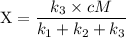
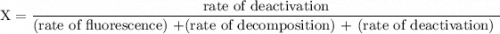
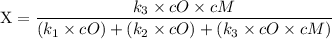
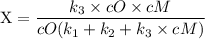
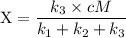 since cM is the concentration of the inert molecule
since cM is the concentration of the inert molecule

