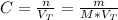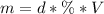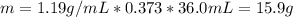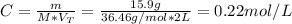Calculate the molarity of a solution made by adding 36.0 mL36.0 mL of concentrated hydrochloric acid ( 37.337.3 % by mass, density 1.19 g/mL1.19 g/mL ) to some water in a volumetric flask, then adding water to the mark to make exactly 2000 mL2000 mL of solution. (It is important to add concentrated acid or base to water, rather than the other way, to minimize splashing and maximize safety.)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:20
Which statement accurately describes the relationship between air pressure, air density, or altitude? as altitude increases, pressure increases.as altitude increases, air density increases.air pressure and density are lowest at sea level.denser air exerts more pressure than less dense air.
Answers: 2

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

You know the right answer?
Calculate the molarity of a solution made by adding 36.0 mL36.0 mL of concentrated hydrochloric acid...
Questions

English, 20.12.2021 14:20

Chemistry, 20.12.2021 14:20


History, 20.12.2021 14:20

Mathematics, 20.12.2021 14:20

Geography, 20.12.2021 14:20

Mathematics, 20.12.2021 14:20



English, 20.12.2021 14:20




Mathematics, 20.12.2021 14:20

History, 20.12.2021 14:20

Mathematics, 20.12.2021 14:20


English, 20.12.2021 14:20

English, 20.12.2021 14:20