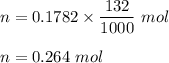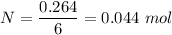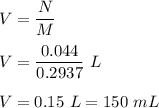Chemistry, 20.09.2020 15:01 genesiloves
Iron in the 2 oxidation state reacts with potassium dichromate to produce Fe3 and Cr3 according to the equation: 6 Fe2 (aq) Cr2O72-(aq) 14 H (aq) <> 6 Fe3 (aq) 2 Cr3 (aq) 7 H2O(l) How many milliliters of 0.2937 M K2Cr2O7 are required to titrate 132.0 mL of 0.1782 M Fe2 solution

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2
You know the right answer?
Iron in the 2 oxidation state reacts with potassium dichromate to produce Fe3 and Cr3 according to t...
Questions


Mathematics, 07.12.2021 23:50

Mathematics, 07.12.2021 23:50


Mathematics, 07.12.2021 23:50


History, 07.12.2021 23:50



Chemistry, 08.12.2021 01:00

Chemistry, 08.12.2021 01:00

Mathematics, 08.12.2021 01:00


Mathematics, 08.12.2021 01:00

Biology, 08.12.2021 01:00

Mathematics, 08.12.2021 01:00

Mathematics, 08.12.2021 01:00

English, 08.12.2021 01:00


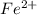 , V = 132 mL .
, V = 132 mL .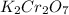 are required to titrate 132.0 mL of 0.1782 M
are required to titrate 132.0 mL of 0.1782 M 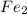 :
: