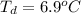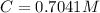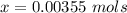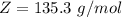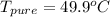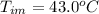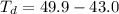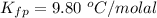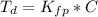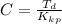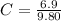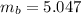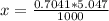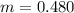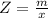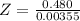Chemistry, 20.09.2020 09:01 davidleew24
Benzophenone freezing point= 49.9 C Benzophenone + unknown freezing point= 43.0 C Benzophenone mass= 5.047 g Unknown mass= .480 g 1. From the difference between the freezing points of the pure benzophenone and the unknown + benzophenone solution, calculate the freezing point depression of the solution. 2. Given the freezing point depression constant for benzophenone, Kfp= 9.80 C/molal, calculate the molality of the solution of unknown in benzophenone. (answer in m) 3. Now use the calculated value for the molality of the solution and the mass of the benzophenone to compute the number of moles of solute present in the solution. 4. Use that calculated number of moles and mass of solute to determine the approximate molar mass (Gram molecular weight) of the unknown solute. (answer in g/mol)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2


Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1
You know the right answer?
Benzophenone freezing point= 49.9 C Benzophenone + unknown freezing point= 43.0 C Benzophenone mass=...
Questions

Mathematics, 20.11.2020 22:40

Mathematics, 20.11.2020 22:40


Mathematics, 20.11.2020 22:40

Mathematics, 20.11.2020 22:40


Mathematics, 20.11.2020 22:40

Mathematics, 20.11.2020 22:40


Biology, 20.11.2020 22:40

Social Studies, 20.11.2020 22:40



Social Studies, 20.11.2020 22:40

Mathematics, 20.11.2020 22:40


Mathematics, 20.11.2020 22:40



Computers and Technology, 20.11.2020 22:40