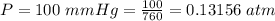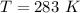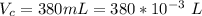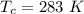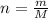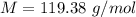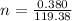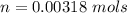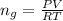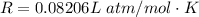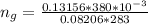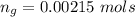Chemistry, 20.09.2020 18:01 juanitarodrigue
The vapor pressure of liquid chloroform, CHCl3, is 100. mm Hg at 283 K. A 0.380 g sample of liquid CHCl3 is placed in a closed, evacuated 380. mL container at a temperature of 283 K.
Assuming that the temperature remains constant, will all of the liquid evaporate? yes/no
What will the pressure in the container be when equilibrium is reached? mm Hg

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which sentence best describes the formation of igneous rock? a- lava on the surface dries up and makes arock b_melted rocks cools and forms crystals c_rocks under tremendous heat and pressure d_magma is melted rock underground
Answers: 1

Chemistry, 21.06.2019 22:00
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1
You know the right answer?
The vapor pressure of liquid chloroform, CHCl3, is 100. mm Hg at 283 K. A 0.380 g sample of liquid C...
Questions



Biology, 12.10.2020 08:01



Spanish, 12.10.2020 08:01

History, 12.10.2020 08:01

Mathematics, 12.10.2020 08:01

Health, 12.10.2020 08:01









Mathematics, 12.10.2020 08:01

Mathematics, 12.10.2020 08:01