

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3


Chemistry, 22.06.2019 23:30
The sum of the oxidation numbers in a neutral compound is always
Answers: 2
You know the right answer?
For the reaction 3C2H2(g)---> C6H6(l) at 25 C the standard enthalpy change is -631 kj and the sta...
Questions


Health, 16.12.2020 18:50

Computers and Technology, 16.12.2020 18:50

Mathematics, 16.12.2020 18:50

Mathematics, 16.12.2020 18:50


Arts, 16.12.2020 18:50





Social Studies, 16.12.2020 18:50




Mathematics, 16.12.2020 18:50

Geography, 16.12.2020 18:50


English, 16.12.2020 18:50

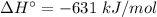 .
.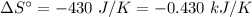 .
.![\Delta G^o=\Delta H^o-T\Delta S^o\\\\\Delta G^o=-631-[298\times (-0.430)]\ kJ\\ \\\Delta G^o=-631-(-128.14)\ kJ\\\\\Delta G^o=-502.86\ kJ](/tpl/images/0770/6490/cfc98.png)


