Chemistry, 20.09.2020 14:01 Serenitybella
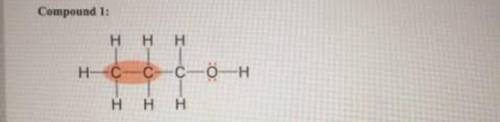
Describe each highlighted bond in terms of the overlap of atomic orbitals. (If the highlighted bond is not a pi bond, select the blank option from the dropdown menu.) Compound 1: Molecular orbital type: Atomic orbitals in the sigma bond: Atomic orbitals in the pi bond: Compound 2: Molecular orbital type: Atomic orbitals in the sigma bond: Atomic orbitals in the pi bond:

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
What is a scientific theory? a. a scientist's guess about how something works b. the results of an experiment obtained using the scientific method c. a proven fact that will never change d. an idea that is backed by data from many sources
Answers: 2

Chemistry, 21.06.2019 20:30
Which of the following true? a_volcanoes and earthquakes often near the plate boundaries. b_volcanoes occur whereve there are tall mountains. c_earthquakes cause volcanoes in the same location to erupt violently d_volcanoes and earthquakes occur only where plates are colliding with each other
Answers: 2


Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
You know the right answer?
Describe each highlighted bond in terms of the overlap of atomic orbitals. (If the highlighted bond...
Questions


Mathematics, 29.05.2021 03:20

Mathematics, 29.05.2021 03:20


Mathematics, 29.05.2021 03:20


Mathematics, 29.05.2021 03:20

Physics, 29.05.2021 03:20

Mathematics, 29.05.2021 03:20

Mathematics, 29.05.2021 03:20


Mathematics, 29.05.2021 03:20

Mathematics, 29.05.2021 03:20

Social Studies, 29.05.2021 03:20


Mathematics, 29.05.2021 03:20

Social Studies, 29.05.2021 03:20


Law, 29.05.2021 03:20

Chemistry, 29.05.2021 03:20