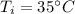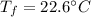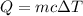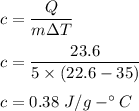Chemistry, 20.09.2020 05:01 jaymoney0531
If 5.0 g of copper cools from 35.0oC to 22.6 oC and loses 23.6 J of heat, what is the specific heat of copper? a. 0.038 J/(g. oC) b. 0.62 J/(g. oC) c. 0.076 J/(g. oC) d. 0.38 J/(g. oC)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Consider the point on the plot where 10.0 g of naoh have been added. what amount of naoh, in moles, has been added? 0.308 mol fecl3 initially present
Answers: 1

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2
You know the right answer?
If 5.0 g of copper cools from 35.0oC to 22.6 oC and loses 23.6 J of heat, what is the specific heat...
Questions

Mathematics, 19.05.2020 13:01

Mathematics, 19.05.2020 13:01









Mathematics, 19.05.2020 13:01



Mathematics, 19.05.2020 13:01



Biology, 19.05.2020 13:01


Computers and Technology, 19.05.2020 13:01

Mathematics, 19.05.2020 13:01