
Chemistry, 20.09.2020 05:01 chewygamerz
HELP!!
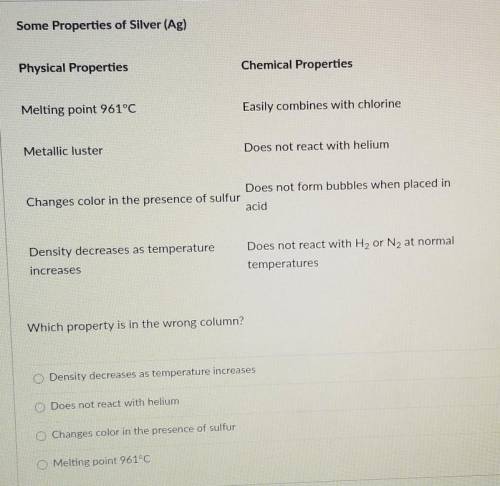
Cade missed chemistry class because he was ill. He borrowed some notes from his lab partner to review what he missed. As Cade was copying the notes, he noticed a mistake in his partner's table, which is shown below. Some Properties of Silver (Ag) Chemical Properties Physical Properties Easily combines with chlorine Melting point 961°C Does not react with helium Metallic luster Does not form bubbles when placed in Changes color in the presence of sulfur acid Density decreases as temperature increases Does not react with H2 or N2 at normal temperatures
Which property is in the wrong column?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 03:30
Nanotechnology, the field of trying to build ultrasmall structures one atom at a time, has progressed in recent years. one potential application of nanotechnology is the construction of artificial cells. the simplest cells would probably mimic red blood cells, the body's oxygen transporters. for example, nanocontainers, perhaps constructed of carbon, could be pumped full of oxygen and injected into a person's bloodstream. if the person needed additional oxygen-due to a heart attack perhaps, or for the purpose of space travel-these containers could slowly release oxygen into the blood, allowing tissues that would otherwise die to remain alive. suppose that the nanocontainers were cubic and had an edge length of 24 nanometers. part a part complete what is the volume of one nanocontainer? (ignore the thickness of the nanocontainer's wall.) express your answer using two significant figures. v v = 1.4ă—10â’20 l previous answers correct significant figures feedback: your answer 1.3824â‹…10â’20 = 1.382ă—10â’20 l was either rounded differently or used a different number of significant figures than required for this part. if you need this result for any later calculation in this item, keep all the digits and round as the final step before submitting your answer. part b suppose that each nanocontainer could contain pure oxygen pressurized to a density of 81 g/l . how many grams of oxygen could be contained by each nanocontainer?
Answers: 3

Chemistry, 23.06.2019 03:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 23.06.2019 05:40
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1
You know the right answer?
HELP!!
Cade missed chemistry class because he was ill. He borrowed some notes from his lab partner...
Questions

History, 02.05.2021 08:40


Mathematics, 02.05.2021 08:40






Chemistry, 02.05.2021 08:50


Computers and Technology, 02.05.2021 08:50

Mathematics, 02.05.2021 08:50


English, 02.05.2021 08:50

History, 02.05.2021 08:50

Health, 02.05.2021 08:50

Mathematics, 02.05.2021 08:50

Computers and Technology, 02.05.2021 08:50

Mathematics, 02.05.2021 08:50

Mathematics, 02.05.2021 08:50



