
Chemistry, 20.09.2020 05:01 tannerbc16
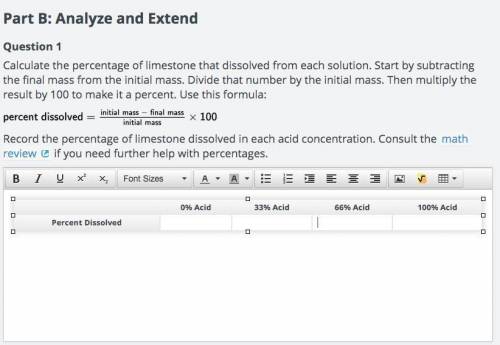
Part B: Analyze and Extend Question 1 Calculate the percentage of limestone that dissolved from each solution. Start by subtracting the final mass from the initial mass. Divide that number by the initial mass. Then multiply the result by 100 to make it a percent. Use this formula:

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Alarge marble is dropped in a graduated cylinder with 35ml of water in it.the water level increases to 49ml.what is the volume of the marble
Answers: 1

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
You know the right answer?
Part B: Analyze and Extend Question 1 Calculate the percentage of limestone that dissolved from each...
Questions






SAT, 30.12.2021 07:10



Mathematics, 30.12.2021 07:10

SAT, 30.12.2021 07:10






Mathematics, 30.12.2021 07:10

English, 30.12.2021 07:10

English, 30.12.2021 07:10





