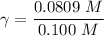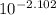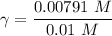Chemistry, 20.09.2020 18:01 jamesgraham577
A. The measured pH of a 0.100 M HCl solution at 25 degrees Celsius is 1.092. From this information, calculate the activity coefficient of H+.B. The measured pH of a solution of 0.010 HCl and 0.090 KCl at 25 degree Celsius is 2.102. Calculate the activity coefficient of H+ in this solution. C. Why does the pH change in part B relative to that in part A?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

You know the right answer?
A. The measured pH of a 0.100 M HCl solution at 25 degrees Celsius is 1.092. From this information,...
Questions

Chemistry, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30


Mathematics, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

Health, 18.03.2021 01:30



Business, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

English, 18.03.2021 01:30

Biology, 18.03.2021 01:30


Business, 18.03.2021 01:30


Mathematics, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

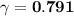
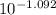
 × C
× C![\gamma = \dfrac{[a]}{C}](/tpl/images/0773/2720/33e10.png)