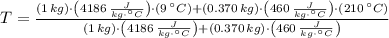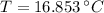Chemistry, 19.09.2020 01:01 jesussanchez1445
A 1.00 kg sample of water at 9.00°C is in a calorimeter. You drop a piece of steel with a mass of 0.370 kg at 210°C into it. After the sizzling subsides, what is the final equilibrium temperature (in °C)? (Make the reasonable assumptions that any steam produced condenses into liquid water during the process of equilibration and that the evaporation and condensation don't affect the outcome.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 18:30
The famous scientist galileo galilei did several experiments with sloping planes, which he rolled metal balls down so that he could study motion. by changing the slope, he could study how the speed at which the ball rolled was affected. what was the independent variable in galileo's experiment? a. the speed of the ball b. the slope of the plane c. whether the ball moved d. what the ball was made of
Answers: 2

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1
You know the right answer?
A 1.00 kg sample of water at 9.00°C is in a calorimeter. You drop a piece of steel with a mass of 0....
Questions


Mathematics, 20.09.2019 03:00

Business, 20.09.2019 03:00




Mathematics, 20.09.2019 03:00

Mathematics, 20.09.2019 03:00

Spanish, 20.09.2019 03:00


History, 20.09.2019 03:00

Mathematics, 20.09.2019 03:00




Mathematics, 20.09.2019 03:00


Computers and Technology, 20.09.2019 03:00


Computers and Technology, 20.09.2019 03:00

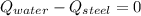
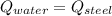
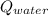 - Heat received by the water sample, measured in joules.
- Heat received by the water sample, measured in joules.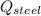 - Heat released by the piece of steel, measured in joules.
- Heat released by the piece of steel, measured in joules.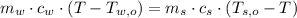
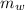 ,
, 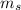 - Masses of the water sample and the piece of steel, measured in kilograms.
- Masses of the water sample and the piece of steel, measured in kilograms.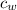 ,
, 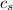 - Specific heat of water and steel, measured in joules per kilogram-Celsius.
- Specific heat of water and steel, measured in joules per kilogram-Celsius.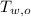 ,
, 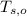 - Initial temperatures of the water sample and the piece of steel, measured in Celsius.
- Initial temperatures of the water sample and the piece of steel, measured in Celsius.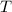 - Final temperature of the sample-piece system, measured in Celsius.
- Final temperature of the sample-piece system, measured in Celsius.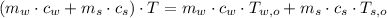
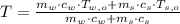
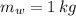 ,
, 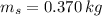 ,
, 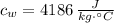 ,
, 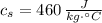 ,
, 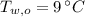 and
and 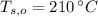 , the final temperature of the system is:
, the final temperature of the system is: