
Chemistry, 10.09.2020 03:01 CoreyHammond1517
When 229.0 J of energy is supplied as heat to 3.00 mol of Ar(g) at constant pressure the temperature of the sample increases by 2.55 K. Assuming that in the experiment the gas behaves as an ideal gas, calculate the molar heat capacities at constant volume and at constant pressure of Ar(g).

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Aphysical reaction is a process in which one or more reactants change into one or more products with different properties. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1
You know the right answer?
When 229.0 J of energy is supplied as heat to 3.00 mol of Ar(g) at constant pressure the temperature...
Questions

History, 14.05.2021 16:20


Chemistry, 14.05.2021 16:20

Mathematics, 14.05.2021 16:20


Medicine, 14.05.2021 16:20



Mathematics, 14.05.2021 16:20




Mathematics, 14.05.2021 16:20






Mathematics, 14.05.2021 16:20

Mathematics, 14.05.2021 16:20

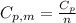
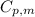 is the molar heat capacity at constant pressure
is the molar heat capacity at constant pressure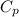 is the heat capacity at constant pressure
is the heat capacity at constant pressure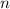 is the number of moles
is the number of moles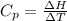
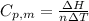
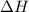 = 229.0 J
= 229.0 J = 2.55 K
= 2.55 K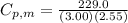
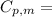 29.93 JK⁻¹mol⁻¹
29.93 JK⁻¹mol⁻¹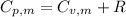
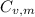 is the molar heat capacity at constant volume
is the molar heat capacity at constant volume 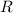 is the gas constant (
is the gas constant (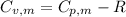
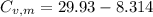
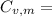 21.62 JK⁻¹mol⁻¹
21.62 JK⁻¹mol⁻¹

