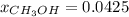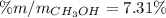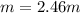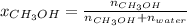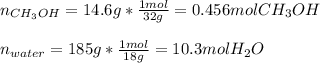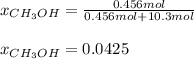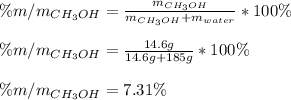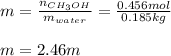Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3


Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2
You know the right answer?
A solution is made containing 14.6g of CH3OH in 185g H2O.1. Calculate the mole fraction of CH3OH.2....
Questions






Biology, 09.12.2019 22:31



Mathematics, 09.12.2019 22:31



Mathematics, 09.12.2019 22:31






Mathematics, 09.12.2019 22:31

Biology, 09.12.2019 22:31

History, 09.12.2019 22:31