
Chemistry, 09.09.2020 08:01 lwilliams28
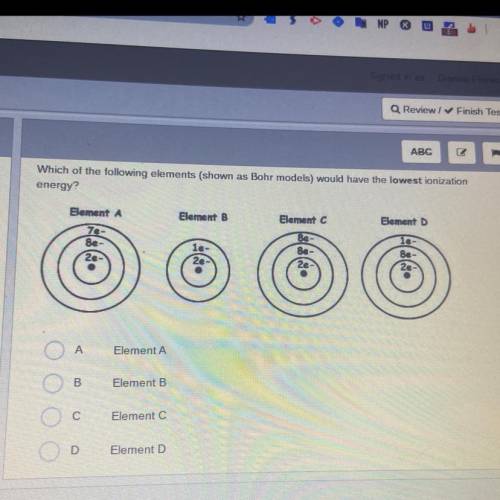
Which of the following elements (shown as Bohr models) would have the lowest ionization
energy?
Element A
Element B
Element a
Element D
ro
save
le-
7e-
8e-
2e
le-
Be
8e
2e
8e-
2e
Circuits
esign
.ce
riods of
device.
table
A
Element A
B
Element B
С
Element C
D
Element D

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 11:30
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
Which of the following elements (shown as Bohr models) would have the lowest ionization
energy?
Questions



Mathematics, 16.10.2019 03:30


Mathematics, 16.10.2019 03:30


History, 16.10.2019 03:30

Mathematics, 16.10.2019 03:30


Health, 16.10.2019 03:30




Business, 16.10.2019 03:30

Physics, 16.10.2019 03:30

Mathematics, 16.10.2019 03:30

Chemistry, 16.10.2019 03:30

Health, 16.10.2019 03:30

Computers and Technology, 16.10.2019 03:30



