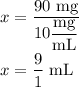Chemistry, 09.09.2020 07:01 itsseand23
If you are administering an insulin dose of 90.0 mg from a bottle at a concentration of 10.0 mg/mL, how many milliliters of the solution do you need to give the patient?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3
You know the right answer?
If you are administering an insulin dose of 90.0 mg from a bottle at a concentration of 10.0 mg/mL,...
Questions


English, 11.12.2020 01:00

Biology, 11.12.2020 01:00


English, 11.12.2020 01:00

Health, 11.12.2020 01:00

History, 11.12.2020 01:00


Mathematics, 11.12.2020 01:00

Social Studies, 11.12.2020 01:00


Law, 11.12.2020 01:00


Mathematics, 11.12.2020 01:00

Chemistry, 11.12.2020 01:00

English, 11.12.2020 01:00


Engineering, 11.12.2020 01:00

Mathematics, 11.12.2020 01:00

English, 11.12.2020 01:00