
Chemistry, 08.09.2020 14:01 awesomegrill
Part AGold-191 undergoes electron capture. Express your answer as a nuclear equation. Part BGold-201 decays to a mercury isotope. Express your answer as a nuclear equation. Part CGold-198 undergoes beta emission. Express your answer as a nuclear equation. Part DGold-188 decays by positron emission. Express your answer as a nuclear equation.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

You know the right answer?
Part AGold-191 undergoes electron capture. Express your answer as a nuclear equation. Part BGold-201...
Questions





Social Studies, 22.10.2020 22:01

Arts, 22.10.2020 22:01

English, 22.10.2020 22:01


Mathematics, 22.10.2020 22:01

Mathematics, 22.10.2020 22:01

Mathematics, 22.10.2020 22:01


History, 22.10.2020 22:01

History, 22.10.2020 22:01




Mathematics, 22.10.2020 22:01


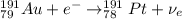
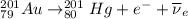
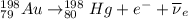
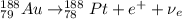
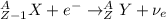
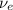 ) is emitted.
) is emitted. 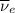 ) are emitted from the Au-201 nucleus and a neutron is converted to a proton (n-1 and Z+1; A remains constant).
) are emitted from the Au-201 nucleus and a neutron is converted to a proton (n-1 and Z+1; A remains constant). 

