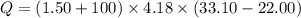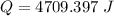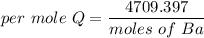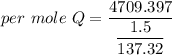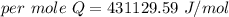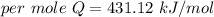Chemistry, 07.09.2020 18:01 breanna7667
When 1.50 g of Ba(s) is added to 100.00 g of water in a container open to the atmosphere, the reaction shown below occurs and the temperature of the resulting solution rises from 22.00°C to 33.10°C. If the specific heat of the solution is 4.18 J/(g ∙ °C), calculate for the reaction, as written. Ba(s) + 2 H2O(l) → Ba(OH)2(aq) + H2(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1

Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3

You know the right answer?
When 1.50 g of Ba(s) is added to 100.00 g of water in a container open to the atmosphere, the reacti...
Questions

Engineering, 02.10.2019 23:30


Physics, 02.10.2019 23:30

Mathematics, 02.10.2019 23:30




English, 02.10.2019 23:30

History, 02.10.2019 23:30

Mathematics, 02.10.2019 23:30


Social Studies, 02.10.2019 23:30


History, 02.10.2019 23:30


Mathematics, 02.10.2019 23:30

Geography, 02.10.2019 23:30


History, 02.10.2019 23:30

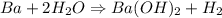
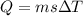
 temperature
temperature