
Chemistry, 06.09.2020 02:01 anthonybowie99
Calculate the mass of each of the following:
a. A sphere of gold with a radius of 10.5 cm. (The volume of a sphere with a radius r is V = (4/3)πr3; the density of gold is 19.3 g/cm^3.)
b. A cube of platinum of edge length 0.021 mm (density = 21.4 g/cm3).
c. 37.3 mL of ethanol (density = 0.798 g/mL).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 01:00
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3
You know the right answer?
Calculate the mass of each of the following:
a. A sphere of gold with a radius of 10.5 cm. (The vol...
Questions




English, 15.01.2020 07:31


Mathematics, 15.01.2020 07:31




Biology, 15.01.2020 07:31


Mathematics, 15.01.2020 07:31



Mathematics, 15.01.2020 07:31

Mathematics, 15.01.2020 07:31


Mathematics, 15.01.2020 07:31

Biology, 15.01.2020 07:31

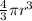
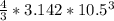 = 4849.68 cm^3
= 4849.68 cm^3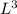 =
= 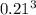 = 9.26 x 10^9 cm^3
= 9.26 x 10^9 cm^3


