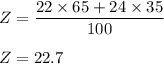Chemistry, 05.09.2020 23:01 jaimevalenzuela60
Consider an element Z that has two naturally occuring isotopes with the following percent abundances: the isotope with a mass number 22.0 is 65.0% abundant; the isotope with a mass number 24.0 is 35.0% abundant. What is the average atomic mass for element Z? Round your answer to the hundredth.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1
You know the right answer?
Consider an element Z that has two naturally occuring isotopes with the following percent abundances...
Questions

Mathematics, 25.09.2019 21:00





Mathematics, 25.09.2019 21:00




Computers and Technology, 25.09.2019 21:00





Biology, 25.09.2019 21:00

Mathematics, 25.09.2019 21:00

Biology, 25.09.2019 21:00

Computers and Technology, 25.09.2019 21:00


Business, 25.09.2019 21:00