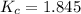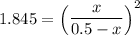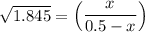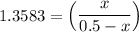Chemistry, 05.09.2020 20:01 Rperez6491
For the reaction:
CO(g) + H2O(g) ⇌ CO2(g) + H2(g)
the value of Kc is 1.845 at a specific temperature. We place 0.500mol CO and 0.500mol H2O in a 1.00L container at this temperature and allow the reaction to reach equilibrium. Determine the equilibrium concentration of all species present in the container.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2


Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3
You know the right answer?
For the reaction:
CO(g) + H2O(g) ⇌ CO2(g) + H2(g)
the value of Kc is 1.845 at a specifi...
the value of Kc is 1.845 at a specifi...
Questions


Mathematics, 02.12.2020 03:10

History, 02.12.2020 03:10




Mathematics, 02.12.2020 03:10


Mathematics, 02.12.2020 03:10

Mathematics, 02.12.2020 03:10

Biology, 02.12.2020 03:10


Mathematics, 02.12.2020 03:10




Mathematics, 02.12.2020 03:10



![K_c = \dfrac{[x][x]}{[0.5-x][0.5-x]}](/tpl/images/0743/5235/b80f7.png)
![K_c = \dfrac{[x]^2}{[0.5-x]^2}](/tpl/images/0743/5235/7f3bb.png)