Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1

You know the right answer?
At a certain temperature, 0.338 mol CH and 0.831 mol HO is placed in a 3.50 L container.
CH(g)+2HO(...
Questions




Chemistry, 05.02.2020 07:51

Mathematics, 05.02.2020 07:51

Mathematics, 05.02.2020 07:51


Mathematics, 05.02.2020 07:51





History, 05.02.2020 07:51

English, 05.02.2020 07:51


History, 05.02.2020 07:51


Mathematics, 05.02.2020 07:51

Mathematics, 05.02.2020 07:51

History, 05.02.2020 07:51

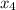 and 0.831 mol H
and 0.831 mol H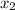 O is placed in a 3.50 L container.
CH
O is placed in a 3.50 L container.
CH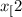 (g) At equilibrium, 6.51 g CO
(g) At equilibrium, 6.51 g CO