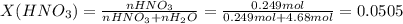Chemistry, 04.09.2020 23:01 0465834wu1
Calculate the mole fraction of nitric acid of a(n) 15.7% (by mass) aqueous solution of nitric acid. Calculate the mole fraction of nitric acid of a(n) 15.7% (by mass) aqueous solution of nitric acid. 2.56×10−2 0.102 5.33×10−2 5.11×10−2 The density of the solution is needed to solve the problem.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Aballoon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon? a balloon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon?
Answers: 3

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2
You know the right answer?
Calculate the mole fraction of nitric acid of a(n) 15.7% (by mass) aqueous solution of nitric acid....
Questions


Mathematics, 23.04.2021 20:00

Mathematics, 23.04.2021 20:00

History, 23.04.2021 20:00



Social Studies, 23.04.2021 20:00


Mathematics, 23.04.2021 20:00

English, 23.04.2021 20:00

Mathematics, 23.04.2021 20:00

Biology, 23.04.2021 20:00

Mathematics, 23.04.2021 20:00

Biology, 23.04.2021 20:00


Advanced Placement (AP), 23.04.2021 20:00




Health, 23.04.2021 20:00