
Chemistry, 03.09.2020 21:01 ilovecatsomuchlolol
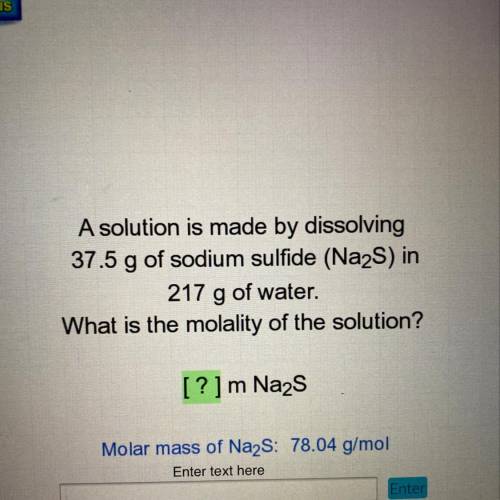
A solution is made by dissolving
37.5 g of sodium sulfide (Na2S) in
217 g of water.
What is the molality of the solution?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Asyringe contains 56.05 ml of gas at 315.1 k. what volume will that gas occupy if the temperature is increased to 380.5 k? a) 12.41 b) 46.42 c) 67.68 d) 81.74
Answers: 1

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2
You know the right answer?
A solution is made by dissolving
37.5 g of sodium sulfide (Na2S) in
217 g of water.
Wha...
217 g of water.
Wha...
Questions

Mathematics, 20.10.2020 20:01


Physics, 20.10.2020 20:01



Mathematics, 20.10.2020 20:01




Mathematics, 20.10.2020 20:01


Geography, 20.10.2020 20:01


Computers and Technology, 20.10.2020 20:01

Mathematics, 20.10.2020 20:01


Mathematics, 20.10.2020 20:01

Computers and Technology, 20.10.2020 20:01

Mathematics, 20.10.2020 20:01



