Chemistry, 02.09.2020 23:01 justhereforanswers13
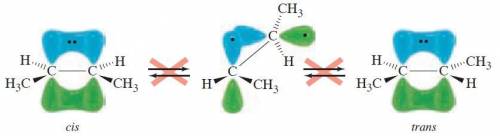
Rotation about a carbon-carbon double bond does not readily occur because: .1) the overlap of the p orbitals of the carbon-carbon π bond would be lost2) the double bond is much shorter and therefore more difficult to rotate3) the overlap of the sp2 orbitals of the carbon-carbon σ bond would be lost4) the double bond is much stronger and therefore more difficult to rotate

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2
You know the right answer?
Rotation about a carbon-carbon double bond does not readily occur because: .1) the overlap of the p...
Questions

Chemistry, 06.04.2021 19:50

Mathematics, 06.04.2021 19:50

World Languages, 06.04.2021 19:50

Chemistry, 06.04.2021 19:50

Mathematics, 06.04.2021 19:50



English, 06.04.2021 19:50

English, 06.04.2021 19:50

Chemistry, 06.04.2021 19:50

Mathematics, 06.04.2021 19:50

Mathematics, 06.04.2021 19:50


Mathematics, 06.04.2021 19:50


Chemistry, 06.04.2021 19:50


Mathematics, 06.04.2021 19:50


English, 06.04.2021 19:50