
Chemistry, 02.09.2020 22:01 shortyyashaun
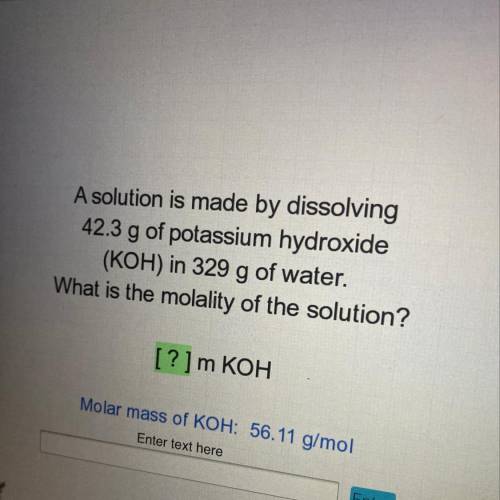
A solution is made by dissolving
42.3 g of potassium hydroxide
(KOH) in 329 g of water.
What is the molality of the solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

You know the right answer?
A solution is made by dissolving
42.3 g of potassium hydroxide
(KOH) in 329 g of water.
...
(KOH) in 329 g of water.
...
Questions


Mathematics, 14.02.2021 19:00

Computers and Technology, 14.02.2021 19:00

Computers and Technology, 14.02.2021 19:00

Biology, 14.02.2021 19:00

Computers and Technology, 14.02.2021 19:00

Mathematics, 14.02.2021 19:00




Chemistry, 14.02.2021 19:00



Mathematics, 14.02.2021 19:00



English, 14.02.2021 19:10



Mathematics, 14.02.2021 19:10



