
Chemistry, 02.09.2020 21:01 heyitzmeamelie
Using the two cell reduction potentials shown for their corresponding reaction, calculate the cell potential for a voltaic cell made from these two systems. A. –1.68 V B. –0.78 V C. 0.78 V D. 1.68 V
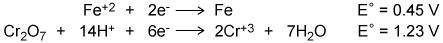

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 10:30
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3

Chemistry, 22.06.2019 22:30
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3
You know the right answer?
Using the two cell reduction potentials shown for their corresponding reaction, calculate the cell p...
Questions

Mathematics, 21.11.2020 05:20

English, 21.11.2020 05:20

English, 21.11.2020 05:20


English, 21.11.2020 05:20



Health, 21.11.2020 05:20


Mathematics, 21.11.2020 05:20

Chemistry, 21.11.2020 05:20

Mathematics, 21.11.2020 05:20

Chemistry, 21.11.2020 05:20



Chemistry, 21.11.2020 05:20


Mathematics, 21.11.2020 05:30

Mathematics, 21.11.2020 05:30

Chemistry, 21.11.2020 05:30



