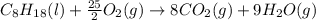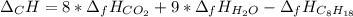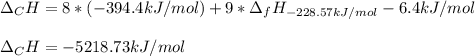Chemistry, 02.09.2020 20:01 zelds63481
Calculate the molar free energy of combustion for liquid n-octane, C8H18 with the given data: AG'CH,,!) 6.4 kJ/mol AG®(00,,8) -394.4 kJ/mol AGʻ(H,0,8) -228.57 kJ/mol a. -5218.7 kJ/mol b. -5384.6 kJ/mol c. -629.4 kJ/mol d. +629.4 kJ/mol e. -2609.4 kJ/mol

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Drag each tile to the correct box arrange the layers not order from oldest to youngest
Answers: 2

Chemistry, 21.06.2019 22:20
One or more substances changing into one or more substances is an example of a
Answers: 1


Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
You know the right answer?
Calculate the molar free energy of combustion for liquid n-octane, C8H18 with the given data: AG'CH,...
Questions



History, 24.03.2020 17:46




English, 24.03.2020 17:46

Mathematics, 24.03.2020 17:46




Mathematics, 24.03.2020 17:47


History, 24.03.2020 17:47



Mathematics, 24.03.2020 17:47