

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
What is the pH of a weak acid that is 0.2% ionized in a 0.2 M solution?...
Questions



Mathematics, 25.02.2021 20:40

Physics, 25.02.2021 20:40

Mathematics, 25.02.2021 20:40

Social Studies, 25.02.2021 20:40


Mathematics, 25.02.2021 20:40


Chemistry, 25.02.2021 20:40


Mathematics, 25.02.2021 20:40


Mathematics, 25.02.2021 20:40


Mathematics, 25.02.2021 20:40

Mathematics, 25.02.2021 20:40


Biology, 25.02.2021 20:40

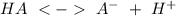
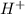 ). If we can to calculate the pH we have to use the equation:
). If we can to calculate the pH we have to use the equation:![pH=-Log[H^+]](/tpl/images/0738/3922/3ca39.png)
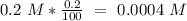
![pH=-Log[0.0004~M]~=~3.40](/tpl/images/0738/3922/1582d.png)


