
Chemistry, 01.09.2020 01:01 Solany6426
1.50
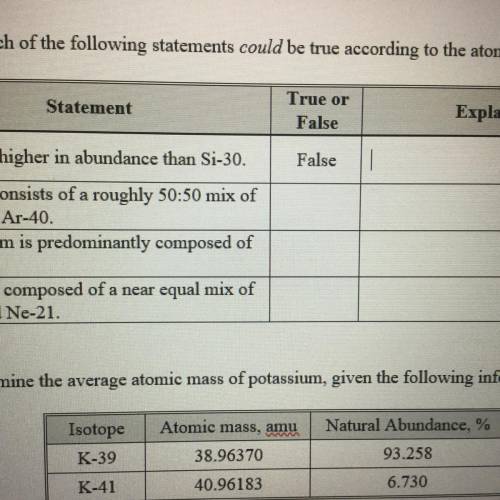
Which of the following statements could be true according to the atomic masses on the periodic table? E
Statement
True or
False
Explanation
False
a. Si-28 is higher in abundance than Si-30.
b. Argon consists of a roughly 50:50 mix of
Ar-36 and Ar-40
c. Strontium is predominantly composed of
Sr-86.
d. Neon is composed of a near equal mix of
Ne-20 and Ne-21

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3

Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1

Chemistry, 23.06.2019 05:30
Based on the formulas, select the compounds below that are covalent: kbr sif4 al2o3 co2 naco3 s7o2 pcl3 fe3n2 h2o s2f10
Answers: 3

Chemistry, 23.06.2019 06:30
The polarity of an oxygen-hydrogen bond is higher than the polarity of a nitrogen-hydrogen bond, allowing amines to be more soluble than alcohols.
Answers: 3
You know the right answer?
1.50
Which of the following statements could be true according to the atomic masses on the periodic...
Questions

History, 07.12.2019 04:31

Mathematics, 07.12.2019 04:31


English, 07.12.2019 04:31

History, 07.12.2019 04:31


















