
Chemistry, 31.08.2020 01:01 kleathers97
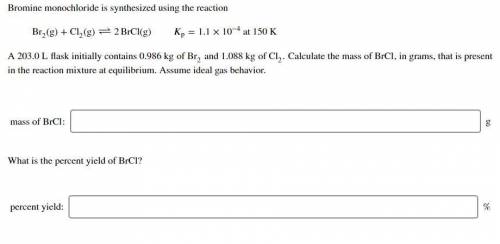
Bromine monochloride is synthesized using the reaction Br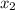 (g)+Cl
(g)+Cl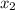 (g)↽−−⇀2BrCl(g) p=1.1×10
(g)↽−−⇀2BrCl(g) p=1.1×10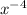 at 150 K A 203.0 L flask initially contains 0.986 kg of Br
at 150 K A 203.0 L flask initially contains 0.986 kg of Br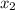 and 1.088 kg of Cl
and 1.088 kg of Cl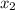 . Calculate the mass of BrCl , in grams, that is present in the reaction mixture at equilibrium. Assume ideal gas behavior. mass of BrCl : _g What is the percent yield of BrCl? percent yield: _%
. Calculate the mass of BrCl , in grams, that is present in the reaction mixture at equilibrium. Assume ideal gas behavior. mass of BrCl : _g What is the percent yield of BrCl? percent yield: _%

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 23.06.2019 01:00
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1

Chemistry, 23.06.2019 03:30
How many grams of sodium chloride are in 250ml of a 2.5m naci solution
Answers: 1
You know the right answer?
Bromine monochloride is synthesized using the reaction Br(g)+Cl(g)↽−−⇀2BrCl(g) p=1.1×10 at 150 K A 2...
Questions





Computers and Technology, 01.01.2021 15:50





Health, 01.01.2021 15:50

Mathematics, 01.01.2021 15:50

Business, 01.01.2021 16:00

Spanish, 01.01.2021 16:00

Mathematics, 01.01.2021 16:00



Mathematics, 01.01.2021 16:00

Biology, 01.01.2021 16:00

Arts, 01.01.2021 16:00

Computers and Technology, 01.01.2021 16:00



