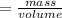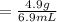Chemistry, 30.08.2020 15:01 briannagiddens
A chemist encounters an unknown metal. They drop the metal into a graduated cylinder containing water, and find the volume change is 6.9 mL. If the metal weighs 4.9 g, what is the density of the metal?

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 06:00
•what conclusions can you make about the relationship between the volume of a gas and its temperature? • what conclusions can you make about the relationship between the volume of a gas and its pressure? • what possible variables have you not accounted for? as you did the procedures, is it possible that the atmospheric pressure may have changed? if it did change over the course of your experiment, then how would your results have been affected?
Answers: 3

Chemistry, 23.06.2019 08:40
Calculate the number of grams of sodium in 3.00 g of each sodium-containing food additive.
Answers: 3

Chemistry, 23.06.2019 13:00
The gram molecular mass or co2 is the same as the gram molecular mass of
Answers: 2

Chemistry, 23.06.2019 14:40
Uuestons niuthe no. of millimoles of hcl required to neutralize 10 ml of 0.2 m na2co3 is(a) 2.0 m mole(b) 4.0 m mole(c) 0.2 m mole(d) 0.4 m mole
Answers: 1
You know the right answer?
A chemist encounters an unknown metal. They drop the metal into a graduated cylinder containing wate...
Questions

Mathematics, 15.04.2021 05:40


Mathematics, 15.04.2021 05:40

Physics, 15.04.2021 05:40

Mathematics, 15.04.2021 05:40



Mathematics, 15.04.2021 05:50

Mathematics, 15.04.2021 05:50

Mathematics, 15.04.2021 05:50


Spanish, 15.04.2021 05:50


Mathematics, 15.04.2021 05:50

Mathematics, 15.04.2021 05:50

Biology, 15.04.2021 05:50


Mathematics, 15.04.2021 05:50