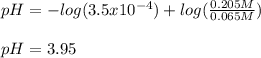Chemistry, 30.08.2020 14:01 jesussanchez1445
What is the pH of a buffer made from 0.130 mol of HCNO (Ka = 3.5 × 10⁻⁴) and 0.410 mol of NaCNO in 2.0 L of solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:40
Listen base your answer to the question on the information below.propane is a fuel that is sold in rigid, pressurized cylinders. most of the propane in a cylinder is liquid, with gas in the space above the liquid level. when propane is released from the cylinder, the propane leaves the cylinder as a gas. propane gas is used as a fuel by mixing it with oxygen in the air and igniting the mixture, as represented by the balanced equation below.c3h8(g) + 5o2(g) → 3co2(g) + 4h2o() + 2219.2 kja small amount of methanethiol, which has a distinct odor, is added to the propane to consumers detect a propane leak. in methanethiol, the odor is caused by the thiol functional group (–sh). methanethiol, ch3sh, has a structure that is very similar to the structure of methanol.what is the correct structural formula for a molecule of methanethiol
Answers: 3

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1


You know the right answer?
What is the pH of a buffer made from 0.130 mol of HCNO (Ka = 3.5 × 10⁻⁴) and 0.410 mol of NaCNO in 2...
Questions



Mathematics, 16.10.2020 16:01


English, 16.10.2020 16:01

Mathematics, 16.10.2020 16:01

Mathematics, 16.10.2020 16:01




Social Studies, 16.10.2020 16:01

Computers and Technology, 16.10.2020 16:01

Mathematics, 16.10.2020 16:01




Mathematics, 16.10.2020 16:01

Chemistry, 16.10.2020 16:01

Mathematics, 16.10.2020 16:01

Health, 16.10.2020 16:01

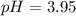
![pH=pKa+log(\frac{[base]}{[acid]} )](/tpl/images/0734/8563/33848.png)
![[base]=\frac{0.410mol}{2.0L}=0.205M](/tpl/images/0734/8563/eabfc.png)
![[acid]=\frac{0.130mol}{2.0L}=0.065M](/tpl/images/0734/8563/e375b.png)