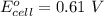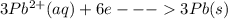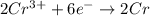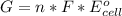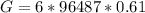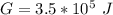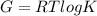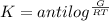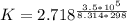Chemistry, 30.08.2020 01:01 yakshp8896
Calculate the equilibrium constant for the reaction: 2 Cr + 3 Pb2+ > 3 Pb + 2 Cr3+ at 25oC. Eocell = 0.61 V

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3
You know the right answer?
Calculate the equilibrium constant for the reaction: 2 Cr + 3 Pb2+ > 3 Pb + 2 Cr3+ at 25oC. Eocel...
Questions

Mathematics, 27.12.2019 05:31

Mathematics, 27.12.2019 05:31


Mathematics, 27.12.2019 05:31

History, 27.12.2019 05:31

History, 27.12.2019 05:31

Physics, 27.12.2019 05:31


Mathematics, 27.12.2019 05:31



Mathematics, 27.12.2019 05:31

Social Studies, 27.12.2019 05:31






Mathematics, 27.12.2019 05:31

Social Studies, 27.12.2019 05:31

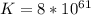
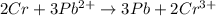
![T = 25^oC = 298 K [room \ temperature ]](/tpl/images/0734/5258/bbcd6.png)