
Chemistry, 29.08.2020 07:01 Jackiebear4593
Which of the following represents an electron configuration that corresponds to the valence electrons of an element for which there is an especially large jump between the second and third ionization energies?
A. 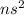
B. 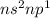
C. 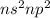
D. 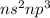

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 23.06.2019 00:00
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1
You know the right answer?
Which of the following represents an electron configuration that corresponds to the valence electron...
Questions





Social Studies, 28.07.2019 22:00

Chemistry, 28.07.2019 22:00


Chemistry, 28.07.2019 22:00


Computers and Technology, 28.07.2019 22:00







Biology, 28.07.2019 22:00

Health, 28.07.2019 22:00


Social Studies, 28.07.2019 22:00



