
Chemistry, 29.08.2020 06:01 maddison788
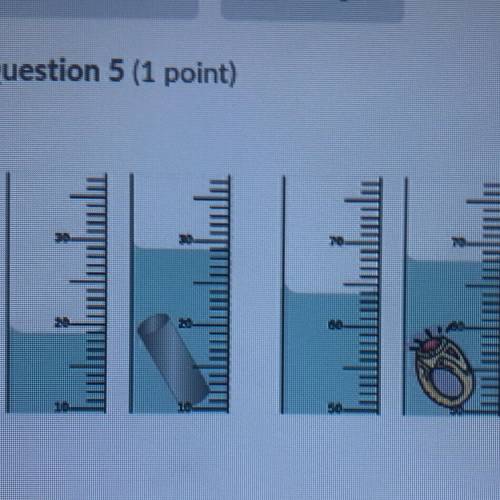
Looking at the picture of the RING above, what is the density of the RING If the
mass of the ring is 15.60g?
.256 g/ml
229 g/ml
3.90 g/ml
01.77g/mL

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2


Chemistry, 22.06.2019 20:00
Listenbase your answer to the question on the information below.nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. an external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body.cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. one hospital keeps a 100.0-gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment.which choice represents the correct product for the beta decay of the co-60? fe-60ni-60fe-61ni-61
Answers: 2

Chemistry, 23.06.2019 04:00
Which of these are physical changes in matter? check all that apply boiling water a pencil being sharpened exploding dynamite freezing water rotting cheese
Answers: 1
You know the right answer?
Looking at the picture of the RING above, what is the density of the RING If the
mass of the ring i...
Questions

Mathematics, 08.12.2019 14:31

Mathematics, 08.12.2019 14:31

Mathematics, 08.12.2019 14:31

Mathematics, 08.12.2019 14:31


Mathematics, 08.12.2019 14:31



Social Studies, 08.12.2019 14:31

History, 08.12.2019 14:31

Biology, 08.12.2019 14:31


Computers and Technology, 08.12.2019 14:31

Business, 08.12.2019 14:31



Mathematics, 08.12.2019 14:31

Mathematics, 08.12.2019 14:31

Mathematics, 08.12.2019 14:31



