
Chemistry, 27.08.2020 21:01 oksanabkrot
A reaction between 7.0 g of copper(II) oxide and 50 mL of 0.20 M nitric acid produces
copper(II) nitrate, Cu(NO3)2 and water.
(c) Determine the limiting reactant.
(d) Calculate the mass of excess reactant after the reaction.
(ANS: 6.6068g)
(e) Determine the percentage yield if the actual mass of copper (II) nitrate obtained from
the reaction is 0.85 g.
(ANS: 90.64%)
How to get the mass of HNO3 from here? I only managed to get mass of NO3 based on the molarity formula. thanks!

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2
You know the right answer?
A reaction between 7.0 g of copper(II) oxide and 50 mL of 0.20 M nitric acid produces
copper(II) ni...
Questions


Mathematics, 18.11.2020 01:50



Social Studies, 18.11.2020 01:50



History, 18.11.2020 01:50




English, 18.11.2020 01:50


Mathematics, 18.11.2020 01:50



Mathematics, 18.11.2020 01:50

Computers and Technology, 18.11.2020 01:50


Mathematics, 18.11.2020 01:50

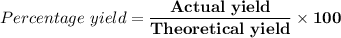
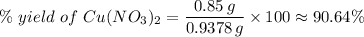
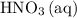 is the limiting reactant.
is the limiting reactant.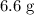 of
of 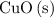 will be in excess.
will be in excess.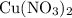 is approximately
is approximately 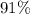 . (Rounded to two significant figures, as in other quantities in the question.)
. (Rounded to two significant figures, as in other quantities in the question.)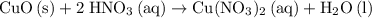 .
.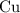 :
: 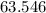 .
. :
: 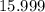 .
.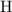 :
: 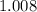 .
.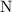 :
: 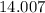 .
.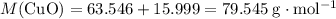 .
.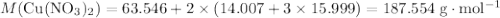 .Limiting Reactant
.Limiting Reactant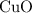 and
and 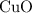 is the limiting one. In other words, assume that all the
is the limiting one. In other words, assume that all the 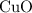 is consumed before
is consumed before 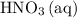 was.
was. 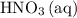 would be required to convert all that
would be required to convert all that 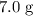 of
of 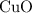 to
to 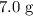 of
of 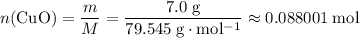 .
.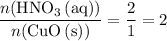 .
.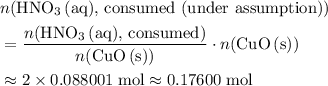 .
. 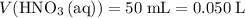 .
.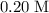 solution:
solution: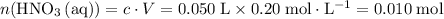 .
.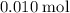 of
of 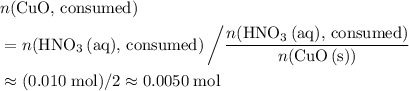 .
.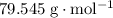 . Therefore, the mass of that
. Therefore, the mass of that 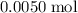 formula units of
formula units of 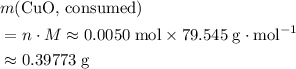 .
.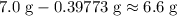 of
of 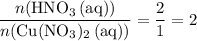 .
.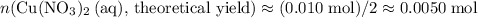 .
.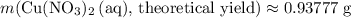 .
.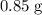 :
: .
.


