Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:20
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3


Chemistry, 23.06.2019 06:20
An object of mass 10.0 kg and volume 1000 ml and density 10 g/ml sinks in water who’s density is 1.0 g/ml. what is the mass of the water which has been displaced in kilograms
Answers: 1
You know the right answer?
Please show some work For the reaction: NO(g) + 1/2 O2(g) → NO2(g) ΔH°rxn is -114.14 kJ/mol. Calcula...
Questions


Mathematics, 15.10.2019 08:10


Physics, 15.10.2019 08:10

English, 15.10.2019 08:10

English, 15.10.2019 08:10


Mathematics, 15.10.2019 08:10




English, 15.10.2019 08:10


Spanish, 15.10.2019 08:10


Mathematics, 15.10.2019 08:10

English, 15.10.2019 08:10

Health, 15.10.2019 08:10

Mathematics, 15.10.2019 08:10

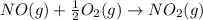
![\Delta H_{rxn}=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0730/9939/db29b.png)
![\Delta H_{rxn}=[(n_{NO_2}\times \Delta H_f_{NO_2})]-[(n_{O_2}\times \Delta H_f_{O_2})+(n_{NO}\times \Delta H_f_{NO})]](/tpl/images/0730/9939/49ca0.png)
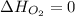 (as heat of formation of substances in their standard state is zero
(as heat of formation of substances in their standard state is zero![-114.14=[(1\times 33.90)]-[(\frac{1}{2}\times 0)+(1\times \Delta H_f{NO})]](/tpl/images/0730/9939/4bbec.png)