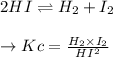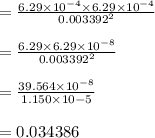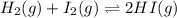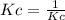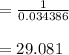Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2
You know the right answer?
A sample of HI (9.30×10^−3mol) was placed in an empty 2.00 L container at 1000 K. After equilibrium...
Questions






Geography, 10.11.2019 03:31

Mathematics, 10.11.2019 03:31

History, 10.11.2019 03:31

Chemistry, 10.11.2019 03:31


History, 10.11.2019 03:31



English, 10.11.2019 03:31


History, 10.11.2019 03:31

Mathematics, 10.11.2019 03:31



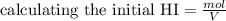
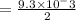
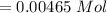
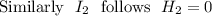
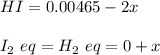
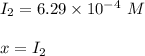
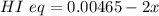
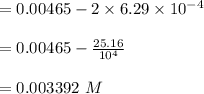
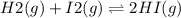 , you can calculate the value of Kc at 1000 K.
, you can calculate the value of Kc at 1000 K.