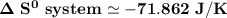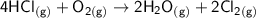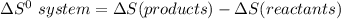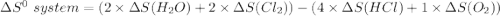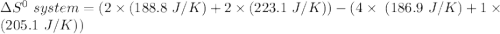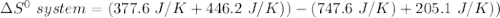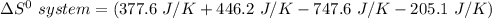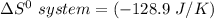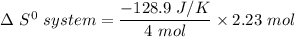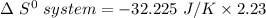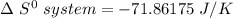Chemistry, 26.08.2020 15:01 rayvingrant16
Consider the reaction: 4HCl(g) + O2(g)2H2O(g) + 2Cl2(g) Using standard absolute entropies at 298K, calculate the entropy change for the system when 2.23 moles of HCl(g) react at standard conditions. S°system = J/K

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 21:50
Answer the questions about this reaction: nai(aq) + cl2(g) → nacl(aq) + i2(g) write the oxidation and reduction half-reactions: oxidation half-reaction: reduction half-reaction: based on the table of relative strengths of oxidizing and reducing agents (b-18), would these reactants form these products? write the balanced equation: answer options: a. 0/na -> +1/na+1e- b. nai(aq) + cl2(g) → nacl(aq) + i2(g) c. +1/na+1e- -> 0 /na d. -1/2i -> 0/i2+2e- e. no f. 4nai(aq) + cl2(g) → 4nacl(aq) + i2(g) g. 2nai(aq) + cl2(g) → 2nacl(aq) + i2(g) h. 4nai(aq) + 2cl2(g) → 4nacl(aq) + 2i2(g) i. nai(aq) + cl2(g) → nacl(aq) + i2(g) j. 0/cl2+2e -> -1/2cl- k. yes
Answers: 1
You know the right answer?
Consider the reaction: 4HCl(g) + O2(g)2H2O(g) + 2Cl2(g) Using standard absolute entropies at 298K, c...
Questions

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

English, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Chemistry, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Chemistry, 18.09.2020 14:01

Social Studies, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Biology, 18.09.2020 14:01

English, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01