
Chemistry, 25.08.2020 20:01 Jennifer16253
What happens to the acidity of solutions A and B after dry ice is added? The pH of solution A decreases, and the pH of solution B increases. The pH of solution A increases, and the pH of solution B decreases. The pH of solution A increases, and the pH of solution B increases. The pH of solution A decreases, and the pH of solution B decreases.
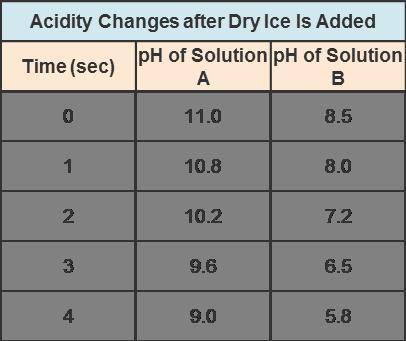

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1
You know the right answer?
What happens to the acidity of solutions A and B after dry ice is added? The pH of solution A decrea...
Questions








Computers and Technology, 22.11.2019 03:31




Mathematics, 22.11.2019 03:31










