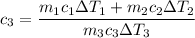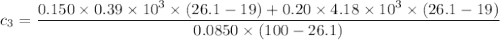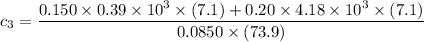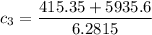Chemistry, 22.08.2020 01:01 Ramone7415
A laboratory technician drops a 0.0850 kg sample of unknown solid material, at a temperature of 100 oC, into a calorimeter. The calorimeter can, initially at 19.0 oC, is made of 0.150 kg of copper and contains 0.20 kg of water. The final temperature of the calorimeter can, and contents is 26.1 oC. Compute the specific heat of the sample.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2

Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1
You know the right answer?
A laboratory technician drops a 0.0850 kg sample of unknown solid material, at a temperature of 100...
Questions









Mathematics, 11.06.2020 16:57

Mathematics, 11.06.2020 16:57

History, 11.06.2020 16:57


Computers and Technology, 11.06.2020 16:57



Social Studies, 11.06.2020 16:57




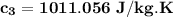
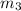 = 0.0850
= 0.0850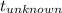 = 100° C
= 100° C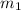 = 0.150 kg
= 0.150 kg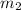 = 0.20 kg
= 0.20 kg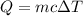
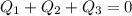
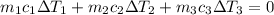
 can be computed by making
can be computed by making