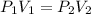Chemistry, 19.08.2020 17:01 mrstealyogirl40
A gas has a volume of 25.0 mL at 2.50 atm. What is the volume at 457 mmHg if the temperature remains constant?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 23.06.2019 03:40
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2
You know the right answer?
A gas has a volume of 25.0 mL at 2.50 atm. What is the volume at 457 mmHg if the temperature remains...
Questions

Mathematics, 13.10.2020 14:01

Health, 13.10.2020 14:01


History, 13.10.2020 14:01





Social Studies, 13.10.2020 14:01


Mathematics, 13.10.2020 14:01

Mathematics, 13.10.2020 14:01

Social Studies, 13.10.2020 14:01

Mathematics, 13.10.2020 14:01



Mathematics, 13.10.2020 14:01

Mathematics, 13.10.2020 14:01

History, 13.10.2020 14:01

Social Studies, 13.10.2020 14:01