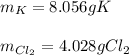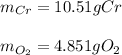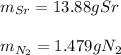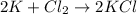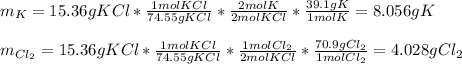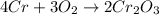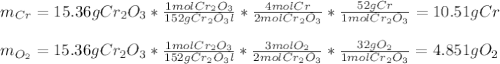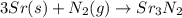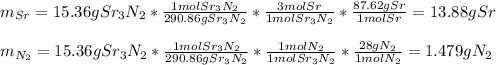Chemistry, 19.08.2020 05:01 momoney5746
For each of the following reactions calculate the mass (in grams) of both the reactants that are required to form 15.39g of the following products.
a. 2K(s) + Cl2(g) → 2Cl(aq)
b. 4Cr(s) + 302(g) → 2Cr2O3(s)
c. 35r(s) + N2(g) → SraNa(s)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1

Chemistry, 23.06.2019 01:30
In which phase of mitosis do the spindle fibers pull the chromosomes apart to opposite sides of the cell ?
Answers: 1

Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 1

Chemistry, 23.06.2019 13:30
The two isotopes of chlorine are 3517cl and 3717cl. which isotope is the most abundant?
Answers: 1
You know the right answer?
For each of the following reactions calculate the mass (in grams) of both the reactants that are req...
Questions

Mathematics, 10.09.2020 15:01

Mathematics, 10.09.2020 15:01

Biology, 10.09.2020 15:01

Mathematics, 10.09.2020 15:01

Geography, 10.09.2020 15:01

Mathematics, 10.09.2020 15:01

Mathematics, 10.09.2020 15:01

Mathematics, 10.09.2020 15:01

Mathematics, 10.09.2020 15:01

Mathematics, 10.09.2020 15:01

Mathematics, 10.09.2020 15:01

Mathematics, 10.09.2020 15:01

Mathematics, 10.09.2020 15:01

Mathematics, 10.09.2020 15:01

Mathematics, 10.09.2020 15:01

Mathematics, 10.09.2020 15:01

Mathematics, 10.09.2020 15:01

Mathematics, 10.09.2020 15:01

Mathematics, 10.09.2020 15:01

Mathematics, 10.09.2020 15:01