
Chemistry, 19.08.2020 03:01 school4life110
A buffer solution is 0.413 M in HF and 0.237 M in KF. If Ka for HF is 7.2×10-4, what is the pH of this buffer solution?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1
You know the right answer?
A buffer solution is 0.413 M in HF and 0.237 M in KF. If Ka for HF is 7.2×10-4, what is the pH of th...
Questions


Computers and Technology, 16.02.2020 19:10

English, 16.02.2020 19:10


Mathematics, 16.02.2020 19:12

Mathematics, 16.02.2020 19:13



Mathematics, 16.02.2020 19:15





Mathematics, 16.02.2020 19:18


Mathematics, 16.02.2020 19:22

Arts, 16.02.2020 19:22

Computers and Technology, 16.02.2020 19:22

Chemistry, 16.02.2020 19:22

Mathematics, 16.02.2020 19:23

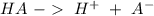
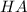 is the acid and
is the acid and 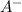 is the base. Additionally, the calculation of the pH of any buffer system can be made with the Henderson-Hasselbach equation:
is the base. Additionally, the calculation of the pH of any buffer system can be made with the Henderson-Hasselbach equation:![pH=pKa~+~Log\frac{[A^-]}{[HA]}](/tpl/images/0724/4288/665aa.png)
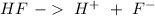
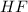 with a concentration of 0.413 M and the base is
with a concentration of 0.413 M and the base is 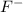 with a concentration of 0.237 M. We can calculate the pKa value if we do the "-Log Ka", so:
with a concentration of 0.237 M. We can calculate the pKa value if we do the "-Log Ka", so: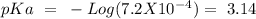
![pH=~3.14~+~Log(\frac{[0.237~M]}{[0.413~M]})~=~2.90](/tpl/images/0724/4288/bcf85.png)


