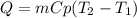Chemistry, 19.08.2020 02:01 aangellexith2885
If the same amount of heat is added to 50.0 g samples of each of the metals which are all at the same temperature, which metal will reach the highest temperature?
Copper 0.385 J/gºC
Magnesium 1.02 J/gºC
Mercury 0.138 J/g °C
Silver 0.237 J/g °C
Lead 0.129 J/gºC
a. Copper
b. Magnesium
c. Mercury
d. Silver
e. Lead

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Look at the reaction below: ca(hco3)2 --> caco3 + co2 + h2o first, balance the reaction. once balanced, use dimensional analysis or another method to find out how many moles of carbon dioxide will be produced if we start with 16.5 moles of calcium bicarbonate (calcium hydrogen carbonate). = mol of co2 number needs to be reported to three significant figures.
Answers: 1


Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2
You know the right answer?
If the same amount of heat is added to 50.0 g samples of each of the metals which are all at the sam...
Questions



English, 10.09.2019 22:20



History, 10.09.2019 22:20

Computers and Technology, 10.09.2019 22:20


Mathematics, 10.09.2019 22:20


Mathematics, 10.09.2019 22:20





Computers and Technology, 10.09.2019 22:20


Computers and Technology, 10.09.2019 22:20

Health, 10.09.2019 22:20