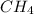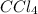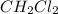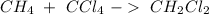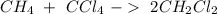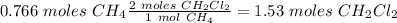Chemistry, 19.08.2020 02:01 ricardorendon100
According to the following reaction, how many moles of dichloromethane (CH2Cl2) will be formed upon the complete reaction of 0.766 moles methane (CH4) with excess carbon tetrachloride? methane (CH4) (g) + carbon tetrachloride (g) → dichloromethane (CH2Cl2) (g)

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 04:31
What is the amount of energy for a photon that has a 125 cm wavelength
Answers: 2


Chemistry, 23.06.2019 08:30
Imagine you are a business executive who wants to pursue an environment policy for your company that limits pollution and uses fewer raw materials but would cost more what might be the discussion to your next broad meeting how would you make your case to your shareholders
Answers: 1
You know the right answer?
According to the following reaction, how many moles of dichloromethane (CH2Cl2) will be formed upon...
Questions



Health, 21.12.2019 18:31


Health, 21.12.2019 18:31

Arts, 21.12.2019 18:31


English, 21.12.2019 18:31


English, 21.12.2019 18:31




Mathematics, 21.12.2019 18:31

Mathematics, 21.12.2019 18:31


Biology, 21.12.2019 18:31


History, 21.12.2019 18:31

Mathematics, 21.12.2019 18:31

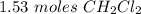 .
.